Training
The insertion of GyneFix requires a unique method of implantation. The single most important prerequisite is to learn to properly insert the anchor.
Read moreThe Women's Health Clinic at UZ Ghent (Belgium) is looking for candidates between 18 and 48 years old for a study that investigates a new insertion method for the copper-containing contraceptive GyneFix via hysteroscopy. More info

Dr Dirk Wildemeersch, MD, PhD (1944-2018) completed his medical training at the University of Ghent, Belgium, and graduated in gynecology in 1976, in South Africa.
Dr. Wildemeersch is the developper of several innovative contraceptive systems, including GyneFix, Gyn-CS, ReLARC, Femilis Cu, Femilis LNG and Fibroplant. For more than 40 years, he was a devoted gynaecologist to his patients and dedicated a substantial part of his time to the clinical development of new IUD concepts and implant systems, originally in collaboration with a group of gynecologists from the University of Ghent, Belgium, under the name IUD-group which later became the International Study Group on Intrauterine Drug Delivery. Under Dr. Wildemeersch’ supervision, a research organization, Contrel Research, was established to manage clinical research and to continue to develop and study innovative drug delivery technologies, aimed at finding improved methods for the prevention and treatment of gynecological disorders, improvements to birth control methods, with higher levels of safety, user acceptability, compliance and quality of life of women. His main topics of research were contraception, including long-acting reversible intrauterine contraception, and intrauterine drug delivery for the treatment of menorrhagia and other gynaecological conditions including postmenopausal treatment. His work is acknowledged by leading research organizations such as the World Health Organization (Reproductive Health Research) in Geneva and by the Population Council in New York.
Following his untimely passing in 2018, his wife, Rina Wildemeersch, has vowed to continue his mission and research.
Development of a new generation copper intrauterine device
The concept of a frameless copper intrauterine device was developed in an effort to create an IUD a contraceptive device that would fit in every uterine cavity. From early on in his praxis, Dr Wildemeersch was convinced of the effectiveness of copper as a contraceptive and a healthy alternative to hormonal contraception such as the pill, which is to this day the most prevalent contraceptive method. Dr Wildemeersch realised that the problems (eg. pain, bleeding, discomfort, …) women were experiencing with traditional copper IUDs were not due to the nature of copper as a contraceptive. Contrary to popular opinion at this time, Dr. Wildemeersch claimed that copper bearing IUD’s were underperforming not due to the copper content, but rather the size and design of the T-shaped IUD relative to size and width of the average woman’s uterus. This conviction led him to develop the first frameless contraceptive copper device IUD, GyneFix.
GyneFix
GyneFix 330 ,available since 1996, was the first CE-approved frameless, anchored intrauterine device. The smaller GyneFix 200 was certified in 2007. GyneFix proved to have significant benefits over T-shaped devices, eliminating complaints such as pain, discomfort and excessive menstrual blood loss, which are the most common causes for discontinuation of the method.
Gyn-CS
Dr Dirk Wildemeersch understood the need for immediate postpartum contraception. He developed a procedure for intracesarean insertion of his frameless, anchored intrauterine device. GYN-CS, is inserted into the uterine cavity immediately following a cesarean section.
ReLARC
Dr. Dirk Wildemeersch understood the need for an insertion method of the frameless, anchored device under direct vision following a number of hysteroscopic procedures or as an alternative to sterilization. The insertion technique was developed by Dr. Wildemeersch in collaboration with German hysteroscopist Dr. med. Thomas Hasskamp.
Fibroplant
Dr. Wildemeersch developed a hormonal version of the frameless, anchored device for patients with therapeutic indications. Fibroplant has significant benefits over T-shaped devices, eliminating complaints such as pain and discomfort.
Femilis Cu & Femilis LNG
Following the development of GyneFix, at the request of the World Health Organisation, Dr. Wildemeersch set out to develop a framed device, focussed on safety and ease of insertion, ideal for settings where ultrasound is not routinely available.
Intrauterine systems: a frameless future?
Why perimenopausal women should consider to use a levonorgestrel intrauterine system
The challenge to solve the expulsion problem of immediate postplacental insertion of IUD
How to become proficient with insertion of frameless IUDs?
Advantages of frameless intrauterine device and system in nulliparous and adolescent women
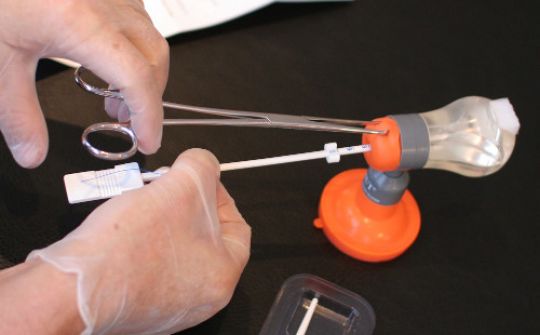
The insertion of GyneFix requires a unique method of implantation. The single most important prerequisite is to learn to properly insert the anchor.
Read more
Our research has produced innovations around IUD contraception. These findings have been integrated in IUD products that are available on the market today.
Read more
Any questions, or want to request a training? Use this form to contact us, and we will get back to you as soon as possible.
Read more